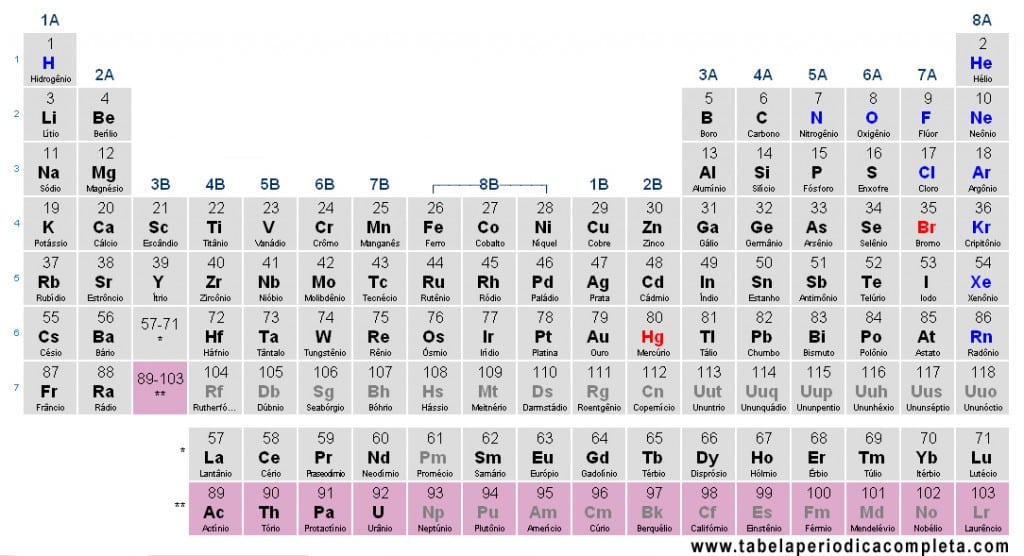
Actinides (or Actinoids) are a series of 15 chemical elements in period 7 of the Periodic Table. They belong to the f-block and are traditionally classified as inner transition metals. The name comes from the first element of the series, Actinium (Ac), and extends to Lawrencium (Lr).
Elements of the Actinide Series
The series ranges from Z = 89 to Z = 103 and includes:
- Actinium (Ac) – 89
- Thorium (Th) – 90
- Protactinium (Pa) – 91
- Uranium (U) – 92
- Neptunium (Np) – 93
- Plutonium (Pu) – 94
- Americium (Am) – 95
- Curium (Cm) – 96
- Berkelium (Bk) – 97
- Californium (Cf) – 98
- Einsteinium (Es) – 99
- Fermium (Fm) – 100
- Mendelevium (Md) – 101
- Nobelium (No) – 102
- Lawrencium (Lr) – 103
Note: the correct symbol for Lawrencium is Lr (not “La”, which is Lanthanum, a Lanthanide).
Position in the Periodic Table
In most representations, Actinides appear in a separate row at the bottom of the Periodic Table, along with the Lanthanides, to keep the table compact. In expanded versions, they may be placed between Radium (Ra) and Rutherfordium (Rf).
Electronic Configuration and Trends
Actinides are characterized by the filling of 5f orbitals. As the atomic number increases, the 5f character becomes more prominent, leading to changes in oxidation states, atomic radius, and magnetic properties. They often show multiple oxidation states (especially +3 and +4, and in some cases +5 and +6).
General Properties
- Metallic nature: silvery metals, relatively dense.
- Radioactivity: all isotopes are radioactive; Thorium and Uranium occur naturally with long half-lives.
- Reactivity: electropositive, forming oxides, halides, and complexes with donor ligands.
- Stability: heavier elements are mostly synthetic, with short half-lives.
Occurrence and Production
Thorium and Uranium are found in minerals such as monazite and uraninite. Transuranic elements (from Np onward) are produced by neutron capture and nuclear reactions in reactors or accelerators, followed by radiochemical separation.
Applications and Importance
- Nuclear energy: Uranium-235 and Plutonium-239 as nuclear fuel and in fission reactions; Thorium studied for the Th–U fuel cycle.
- Neutron sources: Californium-252 in neutron radiography, well logging, and reactor startup.
- Instrumentation: Americium-241 in ionization smoke detectors, as well as research and metrology.
- Scientific research: studies of 5f bonding, complexation, and separation processes (e.g., PUREX and TALSPEAK in nuclear fuel reprocessing).
Safety and Environmental Aspects
Because of their radioactivity and radiotoxic potential, handling requires shielding, containment, and radiological protection protocols. The management of radioactive waste is a central aspect of industrial use and research involving Actinides.
FAQ – Frequently Asked Questions about Actinides
What are the Actinides?
They are 15 elements in period 7, from Actinium (Ac) to Lawrencium (Lr), belonging to the f-block of the Periodic Table.
Are all Actinides radioactive?
Yes. All of them have radioactive isotopes; some, like Thorium and Uranium, have long half-lives and occur naturally, while the heavier ones are synthetic.
Where are Actinides located in the Periodic Table?
They usually appear in a separate row at the bottom of the table, alongside the Lanthanides, to keep the Periodic Table compact.
What are the main applications?
Nuclear energy (fuel and research), neutron sources (Californium-252), smoke detectors (Americium-241), and studies in nuclear chemistry and materials science.
What is the difference between Actinides and Lanthanides?
Both belong to the f-block, but Actinides fill 5f orbitals and are highly radioactive, while Lanthanides fill 4f orbitals and are mostly non-radioactive.
Is “Lawrencium (La)” correct?
No. The correct symbol is Lr. “La” is Lanthanum, which is a Lanthanide.