Periodic properties are trends or characteristics that certain chemical elements follow and that mark their location in the periodic table.
Chemical elements are organized according to their periodic properties, and these properties change with the atomic number. The main periodic properties are: Atomic radius, Ionization energy, Electron affinity, Electronegativity, Electropositivity, and Ionization potential.
Atomic radius
Atomic radius refers to the size of the atom. The greater the number of energy levels, the larger the atom will be. An atom with a greater number of protons exerts a stronger attraction on its electrons.
In other words, atomic radius is the distance from the nucleus of an atom to its electron cloud at the outermost shell. However, since the atom is not rigid, the average atomic radius is calculated as half the distance between the centers of the nuclei of two atoms of the same element in a chemical bond in the solid state.
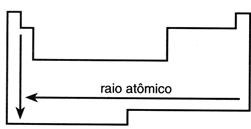
The atomic radius increases from top to bottom within a family of the periodic table, tracking the number of shells of each element’s atoms, and from right to left across the periods of the periodic table.
The higher an element’s atomic number within a period, the stronger the forces between the nucleus and the electron cloud, resulting in a smaller atomic radius.
The element with the largest atomic radius is cesium.
Ionization Energy
Ionization energy is the energy required to remove one or more electrons from an isolated atom in the gaseous state. The size of the atom affects its ionization energy: if the atom is large, its ionization energy will be lower.
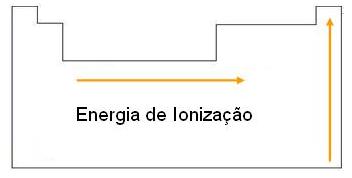
– Within the same family, the energy increases from bottom to top;
– Within the same period, ionization energy increases from left to right.
Electron affinity
Electron affinity is the energy released when an atom in the gaseous state (isolated) captures an electron. The smaller the radius, the greater its electron affinity, within a family or period.
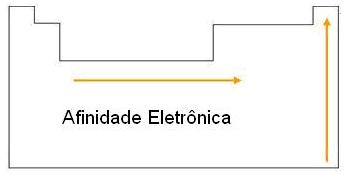
Electron affinity measures the energy released by an atom in the ground state and in the gaseous state upon receiving an electron. It is the minimum energy required to remove an electron from an anion of a given element.
In the noble gases, electron affinity is not significant; however, since adding an electron to any element releases energy, the electron affinity of noble gases is not zero.
Electron affinity behaves similarly to electronegativity, as its increase across the table is not sharply defined: it increases from bottom to top and from left to right.
The chemical element with the highest electron affinity is chlorine.
Electronegativity
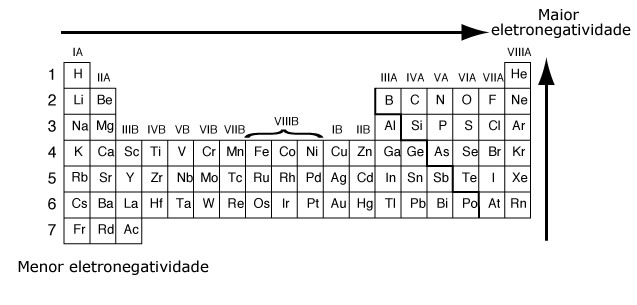
Electronegativity is the attractive force exerted on the electrons in a bond. In the periodic table, electronegativity increases from bottom to top and from left to right.
This property is related to atomic radius: the smaller an atom, the greater the attractive force on electrons.
It is not possible to calculate the electronegativity of a single (isolated) atom, because electronegativity is the tendency of an atom to attract electrons in a covalent bond. Therefore, chemical bonds are needed to measure this property.
According to the Pauling scale*, electronegativity increases in a family from bottom to top—along with the decrease in atomic radius and the increase in interactions between the nucleus and the electron cloud—and across a period from left to right, tracking the increase in atomic number.
The most electronegative element in the periodic table is fluorine.
*The Pauling scale is an empirically constructed and widely used scale in chemistry. It measures the attraction an atom exerts on external electrons in covalent bonds—that is, its electronegativity.
Electropositivity
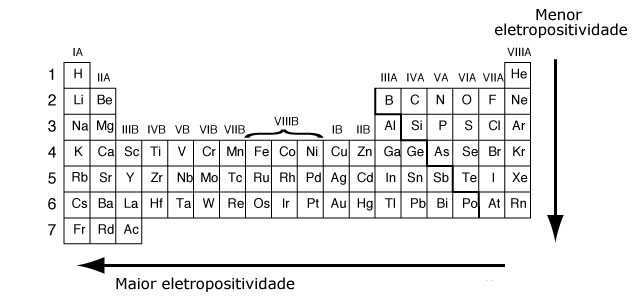
Electropositivity is the tendency of an atom to lose electrons. The higher its value, the greater the metallic character. Atoms with fewer than four valence electrons—metals in general—have a greater tendency to lose electrons and therefore greater electropositivity. An increase in the number of shells reduces the nucleus’s attractive force on peripheral electrons, facilitating electron loss by the atom and consequently increasing its electropositivity.
Electropositivity increases from right to left across periods and from top to bottom within families.
The way to measure a element’s electropositivity is the same as for electronegativity: through chemical bonding. However, the trend is the opposite, since it measures an atom’s tendency to lose electrons. Metals are the most electropositive, and noble gases are excluded¹ because they have no tendency to lose electrons.
The most electropositive chemical element is francium. It has a maximum tendency toward oxidation.
¹Because noble gases are very inert, electronegativity and electropositivity values are not usually studied due to the difficulty of obtaining such data.
Ionization Potential
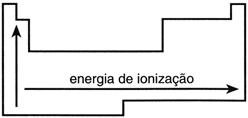
This is the energy required to remove an electron from an isolated atom in the gaseous state. As the size of the atom increases, it becomes easier to remove an electron from the valence shell. Therefore, the larger the atom, the lower the ionization potential.
Ionization potential measures the opposite of electron affinity: the energy required to remove an electron from a neutral atom in the ground state and in the gaseous state. Removing the first electron will use a greater amount of energy than removing the second, and so on.
It behaves the same way as electron affinity and electronegativity; therefore, fluorine and chlorine are the elements with the highest ionization potentials in the periodic table.