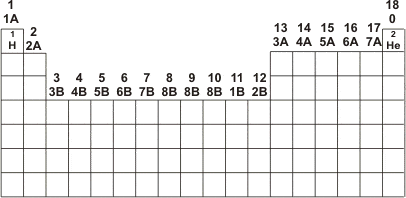
Unlike the periods, the Periodic Table families are arranged vertically in 18 columns. The chemical elements located in the same column of the Periodic Table are considered to belong to the same family because they have similar physical and chemical properties. These elements are part of the same group because they share the same electron configuration in the outermost shell (valence shell).
The numbering of the Periodic Table families starts at 1A (shown in our periodic table as number 1) and continues up to zero or 8A (shown in our periodic table as number 18). There is also Family B.
The Periodic Table is divided into Metals, Nonmetals, and Semimetals, and their families are:
- Family 1A (Group 1): Alkali Metals
- Family 2A (Group 2): Alkaline Earth Metals
- Family B (Groups 3 to 12): Transition Metals
- Family 3A (Group 13): Boron Family
- Family 4A (Group 14): Carbon Family
- Family 5A (Group 15): Nitrogen Family
- Family 6A (Group 16): Chalcogens
- Family 7A (Group 17): Halogens
- Family 0 or 8A (Group 18): Noble Gases
The first group is known as the alkali metals (with the exception of Hydrogen (H)), and the second family is the alkaline earth metals. The set formed between group 3 and group 12, or Family B, is called the transition metals. After this set comes group 13, known as the boron family, followed by the carbon family (Group 14) and the nitrogen family (Group 15). Group 16 is known as the chalcogens, group 17 as the halogens, and finally group 18, which comprises the noble gases.
The last two rows of the Periodic Table represent the lanthanide families and the actinide families, as can be seen in our Complete Periodic Table.