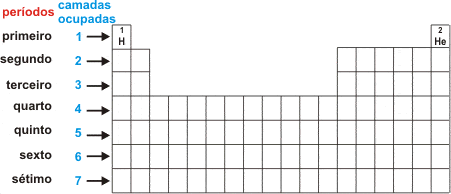
The Periodic Table of the Chemical Elements has horizontal rows, each representing a period (series). The Periodic Table has seven periods and, depending on the level (series) in which the elements are found, the number of electron shells is revealed.
For example, the elements oxygen and fluorine are in the second period and have two electron shells; potassium and calcium are in the fourth period and have four electron levels (K, L, M, N), and so on.
All the elements in the Periodic Table are arranged in numerical sequence according to their atomic numbers. Excluding the first period (where the elements hydrogen and helium are located), all periods begin with a metal and end with a noble gas. The shortest period has two elements and the longest period has 32 elements.
Also read about the Families of the Periodic Table.