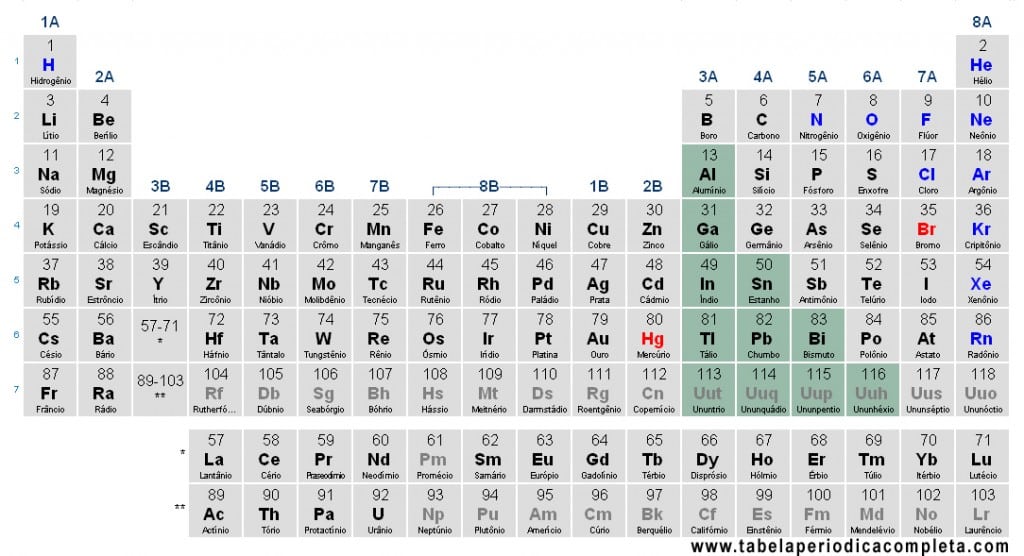
Representative Metals are the metals located in the s- and p-blocks of the Periodic Table (groups 1, 2, and the metallic elements of groups 13–16). They differ from the transition metals (d-block) because their valence electronic configuration ends in ns1–2 or ns2np1–4, which explains much of their chemical behavior.
Electronic configuration of Representative Metals
In these elements, the last energy level shows the following valence electron distribution:
- ns¹ → group 1 (alkali metals)
- ns² → group 2 (alkaline earth metals)
- ns²np¹–np4 → p-block metals (groups 13–16). Note: groups 17–18 are mostly nonmetals and noble gases, therefore not considered representative metals.
Which are the Representative Metals (p-block)
Besides groups 1 and 2, the p-block metals that are part of the Representative Metals include:
- Aluminum (Al)
- Gallium (Ga)
- Indium (In)
- Thallium (Tl)
- Tin (Sn)
- Lead (Pb)
- Bismuth (Bi)
- Polonium (Po) (metal from group 16)
- Nihonium (Nh) – formerly Uut (Z = 113)
- Flerovium (Fl) – formerly Uuq (Z = 114)
- Moscovium (Mc) – formerly Uup (Z = 115)
- Livermorium (Lv) – formerly Uuh (Z = 116)
Naming note: the temporary names Ununtrium (Uut), Ununquadium (Uuq), Ununpentium (Uup), and Ununhexium (Uuh) have been officially replaced by Nihonium (Nh), Flerovium (Fl), Moscovium (Mc), and Livermorium (Lv). These are synthetic elements with very short half-lives and limited experimental data.
General properties and periodic trends
- Metallic character increases down the groups and decreases from left to right across a period.
- Typical oxidation states: Al (+3); Ga/In/Tl (+1/+3, with the inert pair effect stabilizing +1 in heavier elements); Sn/Pb (+2/+4, with +2 increasingly favored in Pb); Bi (+3/+5, with +3 more stable); Po (+2/+4, up to +6).
- Reactivity: alkali and alkaline earth metals are very reactive; in the p-block, reactivity depends on atomic radius, electronegativity, and shielding.
- Amphoterism: Al(OH)₃, Sn(OH)₂/Sn(OH)₄, and Pb(OH)₂ exhibit amphoteric behavior.
- Bonding and structure: many form oxides, halides, hydroxides, and coordination compounds with varied geometries.
Examples, compounds, and applications
- Aluminum (Al): lightweight, corrosion resistant (Al₂O₃ layer); used in alloys, packaging, construction, and electrical conduction.
- Gallium (Ga) and Indium (In): low-melting alloys, semiconductors (GaAs, InP), and display technology (ITO – indium tin oxide).
- Thallium (Tl): used in specialized semiconductors and optical devices; highly toxic, requires strict handling.
- Tin (Sn): alloys (bronze, solder), anticorrosive coatings (tin plating), catalysts, and PVC stabilizers.
- Lead (Pb): lead–acid batteries, radiation shielding; Pb(II) compounds are toxic and environmentally controlled.
- Bismuth (Bi): relatively low toxicity; used in pharmaceuticals (bismuth subsalicylate), fusible alloys, and catalysts.
- Polonium (Po): radioactive, used in heat sources and ionizers under restricted conditions.
Representative Metals × Transition Metals
Although copper (Cu) and zinc (Zn) show some similarities in periodic properties, they are transition metals from the d-block, not part of the representative metals. The determining factor is the occupation of the d subshell in the ground state or in stable oxidation states.
Environmental and safety aspects
Some representative metals and their compounds require caution: Pb and Tl are highly toxic; organotin (Sn) compounds need strict control; Po is radiotoxic. It is essential to follow chemical safety standards, proper waste management, and substitute hazardous materials whenever possible.
FAQ – Frequently Asked Questions about Representative Metals
Which groups form the Representative Metals?
They correspond to the s- and p-blocks of the periodic table: all metals of groups 1 and 2 (alkali and alkaline earth metals) and the metallic elements of groups 13–16.
Why do some elements have two stable oxidation states?
Because of effects like the inert pair effect, ion size, shielding, and electronic stability. For example, Sn (+2/+4) and Pb (+2/+4) show an increasing preference for +2 in heavier atoms.
Are the names Ununtrium/Uuq/Uup/Uuh still correct?
No. The official names are Nihonium (Nh), Flerovium (Fl), Moscovium (Mc), and Livermorium (Lv). These are synthetic elements with short half-lives.
Do Representative Metals form amphoteric compounds?
Yes. Some, such as Al, Sn, and Pb, have amphoteric hydroxides, reacting with both acids and bases.
What is the main difference from transition metals?
Representative metals have valence electrons in the s or p orbitals, with more predictable chemistry based on ionic charge and size. Transition metals (d-block) exhibit multiple oxidation states and rich coordination chemistry.