Before learning how to perform the electron configuration of any neutral atom or ion, it’s essential to understand the basic structure of atoms and the logic behind the distribution of electrons. Below, get to know the concepts of electron shells, energy levels, and sublevels. Then learn how to do electron configuration using the Linus Pauling diagram and how to present it in energy order and geometric order of sublevels. Also learn to identify the outermost sublevel and the most energetic sublevel, and discover the relationship between electron configuration and the periods of the Periodic Table.
Electron shells
Atoms are made up of a nucleus and an electron cloud. The nucleus consists of protons (positively charged particles) and neutrons (neutral particles). The electron cloud consists of electrons (negatively charged particles) that orbit the nucleus. Electrons are arranged in different positions in the electron cloud—some closer to the nucleus, others farther away—forming the so-called electron shells. Theoretically there are infinitely many shells that could be occupied by electrons, but experimentally it has been observed that there are only seven. They are designated by the letters K, L, M, N, O, P, and Q, with K being the first shell, the one closest to the nucleus.
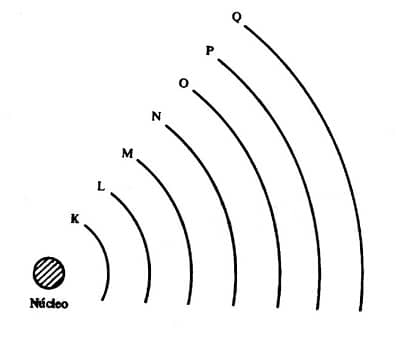
Shells can also be considered energy levels, and in the next section you’ll see why. For now, the key point is that when we choose to use the term “levels,” we must identify them using the numbers 1 to 7, which are called principal quantum numbers (n). The number 1 must be assigned to the level closest to the nucleus. Each energy level (or shell) holds a maximum number of electrons, as shown in the table below:
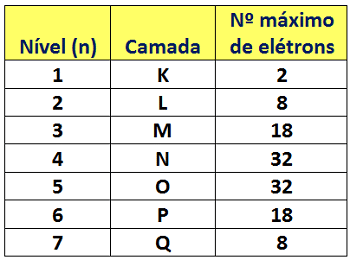
The closer a shell is to the nucleus, the greater the attraction the nucleus exerts on its electrons and the lower the potential energy those electrons have. Conversely, the electrons in shells farther from the nucleus are attracted with less intensity and therefore have higher potential energy. This means that electrons closest to the nucleus—that is, in the innermost shells—are more “tightly bound,” while electrons in the outermost shells are more “free.” To express this “degree of freedom” of electrons relative to the nucleus, we use the concept of energy levels.
Energy levels and sublevels
A level is more energetic the greater the potential energy of the electrons it contains. In other words, a level is more energetic the farther it is from the nucleus. Look at the representation of the electron cloud in the previous section. If we know that the least energetic level of all is 1 (corresponding to the K shell, closest to the nucleus) and the most energetic is 7 (corresponding to the Q shell, farthest from the nucleus), we can conclude that the electrons’ potential energy increases from the innermost to the outermost level of the electron cloud. This rule holds even for atoms that have fewer than seven shells.
The total number of levels an electron cloud has is determined by the number of electrons in the atom. Electrons are distributed according to the maximum capacity of each level, starting with level 1 (K shell) and continuing until all electrons are accommodated. Hydrogen, for example, has only one energy level because it only needs to accommodate one electron. Iron has four levels in its electron cloud, across which 26 electrons are distributed. Uranium, in turn, has seven levels to accommodate its 92 electrons.
But electron configuration isn’t determined only by energy levels. Within the levels, electrons have characteristic amounts of energy. Each of these amounts corresponds to a subdivision of the level, giving rise to the so-called energy sublevels. There are four of them, designated by the lowercase letters s, p, d, f. Like levels, sublevels have quantum numbers that indicate the energy of an electron within them. These are the secondary or azimuthal quantum numbers (ℓ). Respectively, the sublevels s, p, d, f have secondary quantum numbers 0, 1, 2, and 3. Also like levels, each sublevel holds a maximum number of electrons.
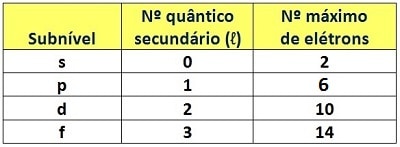
Filling sublevels is done by completely filling one sublevel before moving to the next. However, the occupation of sublevels does not obey the boundaries of levels. Electrons do not simply fill all the sublevels within the same level before moving on; they follow a different sequence—the order of increasing energy. The American chemist Linus Pauling devised a diagram that lets us perform electron configuration according to this increasing order. This tool became known as the Linus Pauling diagram.
Electron configuration using the Linus Pauling diagram
Observe the Linus Pauling diagram. From top to bottom, it shows energy levels in increasing order, represented by the numbers 1 to 7. The sublevels each level has are represented by the letters s, p, d, f. To the right of each letter, a superscript number indicates the maximum number of electrons that sublevel can hold. The arrows indicate the direction in which the diagram should be read. Each arrow should be followed to the end before moving to the beginning of the next one.
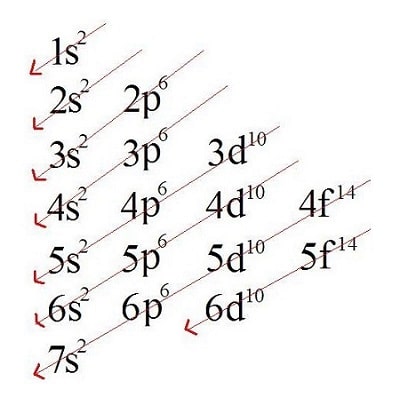
Reading the diagram gives us the increasing order of the energy sublevels, which is as follows:
1s2 – 2s2 – 2p6 – 3s2 – 3p6 – 4s2 – 3d10 – 4p6 – 5s2 – 4d10 – 5p6 – 6s2 – 4f14 – 5d10 – 6p6 – 7s2 – 5f14 – 6d10 – 7p6
This is the order in which electrons occupy the energy sublevels. To perform electron configuration, we must follow this order and observe the maximum number of electrons each sublevel holds. The configuration is done by filling each sublevel before moving to the next. If the last sublevel has fewer electrons than its maximum capacity, that’s fine. In that case, the number accompanying the letter must be replaced by the actual number of electrons.
Let’s look at the example of the electron configuration of a neutral iron atom, which has 26 electrons. According to the arrow order, the configuration is: 1s2 – 2s2 – 2p6 – 3s2 – 3p6 – 4s2 – 3d6. Note that the 3d sublevel, the last to be filled, can hold up to 10 electrons, but there were only 6 to be placed there.
Sublevels in energy order and in geometric order
In the iron example, notice that when we write the sequence of sublevels according to the diagonals of the diagram, we write them exactly in increasing energy order: 1s2 – 2s2 – 2p6 – 3s2 – 3p6 – 4s2 – 3d6. This is the so-called energy order.
The other way to represent electron configuration by energy sublevels is the geometric order. In it, after configuring according to energy order, we group the sublevels of each level. For the neutral iron atom, the geometric order is: 1s2 – 2s2 2p6 – 3s2 3p6 3d6 – 4s2.
Most energetic sublevel and outermost sublevel
The energy order lets us identify the most energetic sublevel, which is always the last in the sequence. For iron, it’s the 3d6 sublevel. The geometric order shows the outermost sublevel, which is also always the last. For iron, the outermost sublevel is 4s2.
Sometimes the most energetic sublevel and the outermost sublevel are the same, but when that happens it’s just a coincidence. It’s always necessary to order the sublevels by energy to find the most energetic one and by geometry to identify the outermost one.
Electron configuration of neutral atoms and ions
The electron configuration of neutral atoms is done by considering the number of electrons the element’s atom has in its ground state, which equals its number of protons or atomic number (Z). That’s why, when we gave the example of the neutral iron atom (Z = 26), we distributed 26 electrons.
The case of the electron configuration of ions isn’t complicated. An ion is simply an atom that has gained or lost electrons from its outermost level (last level). An ion that results from gaining electrons is called an anion, and one formed by losing electrons is called a cation. The easiest way to configure an ion is to first configure its neutral atom, arrange the sublevels in geometric order, and then remove or add electrons from the last level.
See the example of the configuration of the iron cation Fe+2. This is an iron atom that lost 2 electrons from its last level.
Configuration of the neutral iron atom (Z = 26):
Energy order: 1s2 – 2s2 – 2p6 – 3s2 – 3p6 – 4s2 – 3d6
Geometric order: 1s2 – 2s2 2p6 – 3s2 3p6 3d6 – 4s2
We identify the outermost level, which is 4. It has only one sublevel, s2. We need to remove two electrons from the last level, and they will come precisely from the only sublevel that level has. Therefore, the 4s2 sublevel will cease to exist, and the configuration of Fe+2 becomes:
Configuration of the Fe+2 cation:
Geometric order: 1s2 – 2s2 2p6 – 3s2 3p6 3d6
Energy order: 1s2 – 2s2 – 2p6 – 3s2 – 3p6 3d6
Electron configuration in the Periodic Table
There is a relationship between the periods of the Periodic Table and the energy levels that elements have. Note that the Table has seven periods, numbered from top to bottom. The number of each corresponds to the number of levels (or shells) its elements have. Thus, the elements of the first period—hydrogen and helium—have only one energy level, while those of the second period have two levels, and so on up to the seventh period.