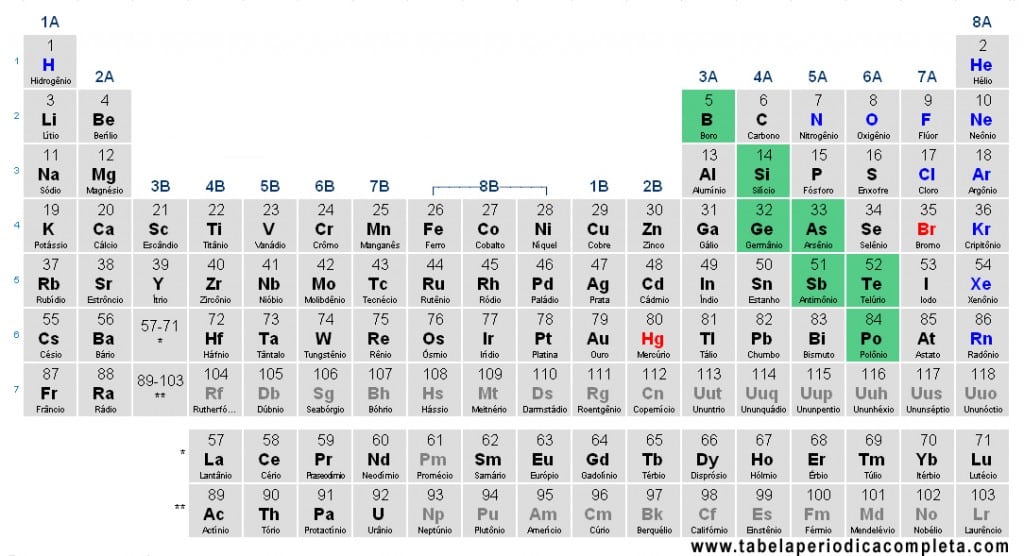
Metalloids, also called Semi-Metals, are chemical elements that present both metallic and nonmetal properties. This duality is reflected in their physical and chemical characteristics, making them a transitional group important for understanding the Periodic Table.
Which are the Metalloids?
According to the most common classification, Metalloids include:
- Boron (B) – group 13 (3A)
- Silicon (Si) – group 14 (4A)
- Germanium (Ge) – group 14 (4A)
- Arsenic (As) – group 15 (5A)
- Antimony (Sb) – group 15 (5A)
- Tellurium (Te) – group 16 (6A)
- Polonium (Po) – group 16 (6A)
Some authors also include Astatine (At) as part of the metalloids, though this inclusion is considered arbitrary.
General properties of Metalloids
- Electrical semiconductors: they can conduct electricity under certain conditions, forming the basis of electronic devices.
- Thermal semiconductors: intermediate heat conductivity, different from good metallic conductors and typical insulators.
- Formation of amphoteric oxides: capable of reacting as acids or bases.
- Electronic structure: they show a slight overlap between the valence band and the conduction band, explaining their semiconductor behavior.
Location in the Periodic Table
In the Periodic Table, metalloids are positioned almost along a diagonal line from Boron (B) to Polonium (Po). Elements to the left of this diagonal are metals, while those to the right are nonmetals. This intermediate position reinforces their hybrid nature.
Technological importance
Metalloids play an essential role in modern applications. Silicon, for example, is the basis of the semiconductor industry, indispensable in the production of computers, smartphones, and virtually all modern electronics. Germanium is used in semiconductors, fiber optics, and sensors. Arsenic and Antimony are employed in alloys and electronic components. Tellurium is used in solar panels and thermoelectric materials.
Current classification
Although the classification of Metalloids is still widely used in schools and textbooks, the International Union of Pure and Applied Chemistry (IUPAC) has never officially recognized it. In Brazil, the Brazilian Chemical Society (SBQ) abandoned the category in 2001. Currently:
- Germanium, Antimony, and Polonium are classified as metals in official tables.
- Boron, Silicon, Arsenic, and Tellurium are considered nonmetals.
Despite this, the term metalloid remains widely used as a didactic tool to better explain the transition between metals and nonmetals.
FAQ – Frequently Asked Questions about Metalloids
Which elements are classified as Metalloids?
The main ones are: Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium, and Polonium. In some classifications, Astatine is also included.
Why are they called Metalloids?
Because they have intermediate properties between metals and nonmetals, in both physical and chemical aspects.
Where are Metalloids located in the Periodic Table?
They are distributed along a diagonal line from Boron (B) to Polonium (Po), separating the metals from the nonmetals.
What are the main applications of Metalloids?
Silicon: semiconductors and microchips. Germanium: fiber optics and electronics. Arsenic and Antimony: alloys and electronic devices. Tellurium: solar energy and thermoelectric materials.
Is the classification of Metalloids still official?
No. Neither the IUPAC nor the SBQ officially recognize this category. However, it is still used in schools and textbooks as a teaching aid.
Why is Silicon the most important Metalloid?
Because it is the main material used in the semiconductor industry, serving as the foundation of modern technology such as computers, smartphones, and digital systems.