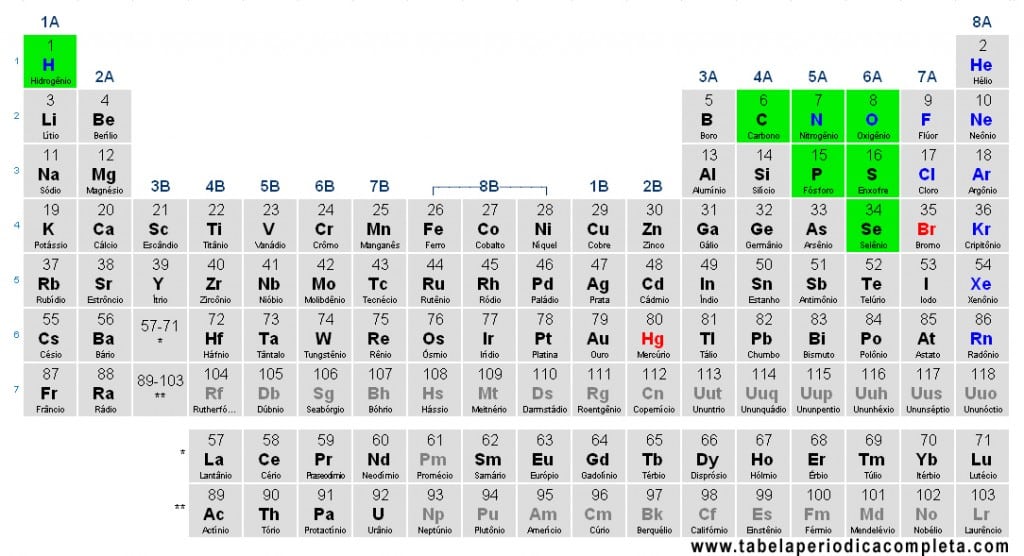
Nonmetals (or Ametals) are chemical elements with high electronegativity and a strong tendency to gain or share electrons, forming primarily covalent bonds. In the Periodic Table, they are located mostly in the upper right corner. The only exception is Hydrogen (H), which is placed at the top of the alkali metals column, but due to its chemical behavior, it is classified as a nonmetal.
Which elements are Nonmetals?
For didactic purposes, the following are considered nonmetals (grouped by families):
- Hydrogen (H)
- Group 14 (carbon group – nonmetallic): Carbon (C)
- Group 15 (pnictogens – nonmetallic): Nitrogen (N), Phosphorus (P)
- Group 16 (chalcogens – nonmetallic): Oxygen (O), Sulfur (S), Selenium (Se)
- Halogens (group 17): Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I) (astatine, At, is radioactive with less known behavior and is sometimes listed separately)
- Noble Gases (group 18): Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn) (and superheavy oganesson, Og, which has no practical applications due to instability)
Note: some older tables listed “only seven” nonmetals (C, N, O, P, S, Se, H), but the modern classification also includes halogens and noble gases.
General properties of Nonmetals
- High electronegativity and electron affinity compared to metals; they tend to form anions (in ionic compounds) or share electrons (in covalent compounds).
- Conductivity: generally poor conductors of heat and electricity. Exceptions: graphite (C) conducts in-plane.
- Physical states at 25 °C: may be gases (H₂, N₂, O₂, F₂, Cl₂, noble gases), liquid (Br₂), or solids (C, P, S, Se, I).
- Allotropy: common among them, e.g. Carbon (diamond, graphite, graphene, fullerenes), Phosphorus (white, red, black), Sulfur (rhombic, monoclinic).
- Oxides are mostly acidic (e.g., CO₂, SO₃, P₂O₅); some are neutral (CO, N₂O) or acid anhydrides that form acids in water.
Chemical bonding and substance types
- Molecular: many exist as discrete molecules (O₂, N₂, Cl₂, H₂O, NH₃, CO₂).
- Covalent networks: solids with high melting points and hardness, such as diamond (C) and forms of silica (SiO₂; Si is a metalloid but bonds covalently with O).
- Ionic compounds: formed when reacting with metals (e.g., NaCl, Ca₃(PO₄)₂), where the nonmetal acts as the anion.
Reactivity and periodic trends
- With metals: form ionic salts (halides, sulfides, oxosalts).
- With other nonmetals: generate a wide range of covalent compounds (acids, Lewis bases, oxoacids, hydrides, oxides).
- Trends: electronegativity and ionization energy increase from left to right and bottom to top; atomic radius decreases in the same direction.
Biological and environmental importance
- Life’s building blocks: biomolecules (proteins, lipids, carbohydrates, nucleic acids) are composed mainly of C, H, O, N, P, and S.
- Biogeochemical cycles: carbon cycle, nitrogen cycle, oxygen cycle, and phosphorus cycle regulate climate, soil fertility, and ecosystems.
- Atmosphere: N₂ and O₂ dominate air composition; noble gases are stable trace components; ozone (O₃) shields against UV radiation.
Applications and practical examples
- Carbon: advanced materials (graphene, nanotubes), electrodes, polymers (petrochemical industry).
- Nitrogen: ammonia production (Haber-Bosch) for fertilizers; inert atmosphere in industries.
- Oxygen: essential for respiration and combustion; steelmaking and oxidation processes.
- Phosphorus: fertilizers (phosphates), detergents, matches (red phosphorus).
- Sulfur: sulfuric acid, rubber vulcanization, pharmaceuticals and agrochemicals.
- Halogens: water disinfection (Cl₂, hypochlorite), pharmaceuticals, halogenated polymers.
- Noble gases: lighting (Ne, Ar, Xe), welding shield gases, helium for cryogenics.
Common misconceptions corrected
- “Only seven nonmetals”: outdated. Modern classification includes halogens and noble gases as well.
- “All are insulators”: generally true, but exceptions exist (graphite conducts electricity in-plane).
- “Their oxides are always acidic”: most are, but some are neutral (CO, NO) and exceptions exist depending on oxidation states.
FAQ – Frequently Asked Questions about Nonmetals
Which are the main nonmetals?
H, C, N, O, P, S, Se, as well as the halogens (F, Cl, Br, I) and noble gases (He, Ne, Ar, Kr, Xe, Rn).
Why is Hydrogen a nonmetal if it is placed with alkali metals?
It is placed in group 1 due to its ns¹ configuration, but its chemical properties (H₂ formation, covalent bonding, acidity) are typical of a nonmetal.
Do nonmetals conduct electricity?
Generally no. Exceptions include graphite (carbon allotrope) which conducts in layers, and some nonmetals can conduct under special conditions (e.g., doping, pressure).
Are all nonmetal oxides acidic?
Most are (e.g., SO₃ → H₂SO₄; P₂O₅ → H₃PO₄), but some are neutral (CO, NO), and others vary depending on oxidation state and structure.
What are the most common uses of nonmetals?
Fertilizers (N, P), water treatment (Cl), polymers (C, H, Cl, F), electronics and lighting (noble gases), oxidation and respiration (O₂).
Are nonmetals the majority on Earth?
In terms of number of elements, metals are more numerous. However, nonmetals dominate the biosphere and the atmosphere (C, H, O, N) and are the foundation of the chemistry of life.