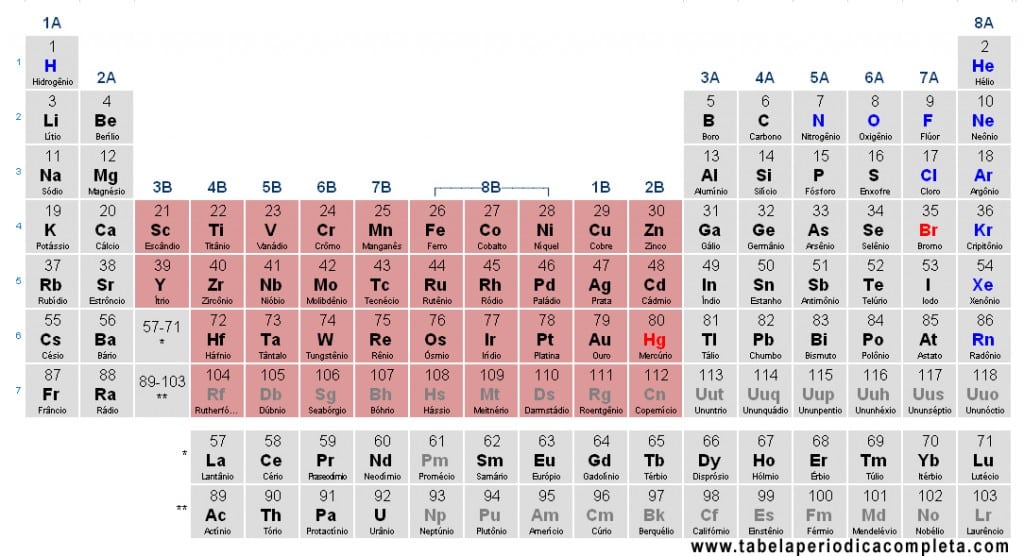
The Transition Metals are defined by the IUPAC as “elements whose atom has an incomplete d subshell or can form cations with an incomplete d subshell.” In the Periodic Table, they correspond to the d-block, covering groups 3 to 12. In a broader didactic sense, group 12 elements (Zn, Cd, Hg) are sometimes included, although under the strict IUPAC definition they are not considered transition metals since their most stable oxidation state has a complete d10 configuration.
External vs. Internal Transition
The Lanthanides (57–71) and Actinides (89–103) are called inner transition elements (f-block). Some authors separate them from “transition metals” due to their unique properties. The “external transition metals” belong to the d-block:
- 1st transition series (3d): Z = 21–30 (Sc to Zn)
- 2nd transition series (4d): Z = 39–48 (Y to Cd)
- 3rd transition series (5d): Z = 72–80 (Hf to Hg)
The term “transition metals” reflects their intermediate position between groups 2 and 13, with the successive addition of electrons to the d subshell.
Electronic Configuration and Trends
In general, their valence configuration can be represented as (n−1)d1–10ns0–2. Filling of the d subshell drives many trends:
- Variable oxidation states (e.g., Fe²⁺/Fe³⁺, Cu⁺/Cu²⁺, Mn ranging from +2 to +7), supporting diverse coordination chemistry.
- Moderate ionization energies and ionic radii that allow multiple complex geometries (octahedral, tetrahedral, square planar).
- Lanthanide contraction: the reduction of ionic radii in the lanthanides affects 4d/5d elements, making many 5d metals denser and with higher melting points.
Characteristic Properties
- Hard metals with high melting/boiling points (exceptions include Hg, which is liquid at room temperature).
- Good conductors of heat and electricity (Cu, Ag, and Au are benchmarks in conductivity).
- Colored complexes and paramagnetism due to d–d electronic transitions and unpaired electrons (explained by crystal field theory / ligand field theory).
- High catalytic activity: their variable oxidation states and surface chemistry facilitate redox cycles and ligand activation.
Reactivity and Coordination Chemistry
Transition metals form a wide variety of complexes with ligands (H₂O, NH₃, halides, CN⁻, CO, phosphines), which affect color, magnetism, and reactivity. The ligand field strength (spectrochemical series) influences the energy splitting of the d orbitals (Δ), determining whether complexes are high-spin or low-spin.
Examples and Applications
- Iron (Fe): basis of steel and alloys; Fe²⁺/Fe³⁺ species play key roles in biology (hemoglobin) and industry.
- Copper (Cu): electrical wiring, alloys (brass, bronze), and catalysts.
- Chromium (Cr): provides corrosion resistance in stainless steels; Cr(VI) compounds are toxic and require careful control.
- Nickel (Ni) and Cobalt (Co): used in batteries, high-performance alloys, and hydrogenation/dehydrogenation catalysts.
- Vanadium (V): catalyst (e.g., V₂O₅ in sulfuric acid production), alloys with high strength.
- Palladium (Pd), Platinum (Pt), Rhodium (Rh): catalysts in fine chemistry, reforming, and automotive catalytic converters.
- Titanium (Ti): lightweight and strong alloys; TiO₂ as a pigment and photocatalyst.
Environmental and Safety Aspects
Some transition metals and their compounds are toxic (e.g., Cr(VI), Cd, Hg). Safe practices include exposure control, regulated disposal, and replacement with safer alternatives when possible.
FAQ – Frequently Asked Questions about Transition Metals
Which groups are the Transition Metals?
They correspond to the d-block of the Periodic Table, groups 3 to 12. In some textbooks, Zn, Cd, and Hg are included; under the strict IUPAC definition, they are not.
Why are many transition metal compounds colored?
Because of d–d electronic transitions and the metal–ligand interaction (crystal field/ligand field), which split the energy levels of the d orbitals.
What is the difference between external and internal transition?
External transition refers to the d-block (groups 3–12). Internal transition refers to the Lanthanides and Actinides (f-block), which have distinct chemical behavior.
Why are transition metals good catalysts?
They have variable oxidation states and coordination sites that activate reactants, stabilize intermediates, and facilitate redox reactions.
What are some industrial applications?
Steels and alloys (Fe, Cr, Ni), conductors (Cu), catalysts (Pt, Pd, Rh, V₂O₅), pigments, and high-performance materials (Ti and its alloys).
Are group 12 elements always transition metals?
Didactically, they may be listed as such, but under the strict IUPAC definition they are not, since they have d10 in their common oxidation state and do not feature an incomplete d subshell as their most stable cation.