Electronic Structure

Hydrogen has the simplest atomic structure of any other chemical element, its nucleus contains one proton with a +1 charge, with a surrounding electron and electron configuration 1s¹. Hydrogen atoms can achieve stability in three different ways:
- By forming a covalent bond (a pair of electrons) with other atoms;
- By losing an electron to form H+;
- By gaining electrons and forming H-;
Position in the Periodic Table
Hydrogen (H) is the first element of the Periodic Table and has unique characteristics.
The electronic structure of the hydrogen atom resembles that of the alkali metals (Group 1), the halogens, and the elements of Group 14.
In the case of similarity with alkali metals, it is due to the much greater tendency of hydrogen to form covalent bonds.
Halogens, on the other hand, generally gain electrons forming negative ions X-. It is not common for hydrogen to form a negative ion, although it does form ionic hydrides M+H- with some highly electropositive metals, justifying its similarity with the electronic structure of halogens.
In some respects, the electronic structure of hydrogen also resembles that of Group 14 elements, since both have a half-filled outer shell.
Hydrogen Abundance
Hydrogen is the most abundant element in the universe. According to estimates, the universe is composed of 92% hydrogen and 7% helium, with all other elements together accounting for only 1%. However, the amount of H2 in Earth’s atmosphere is very small, since the Earth’s gravitational field is too weak to retain such a light element. Nevertheless, some H2 is found in volcanic gases. In contrast, hydrogen is the tenth most abundant element in the Earth’s crust (1520 ppm or 0.152% by weight). It is also found in large quantities in ocean water. Compounds containing hydrogen are very abundant, especially water, living organisms (carbohydrates and proteins), organic compounds, fossil fuels (coal, oil, and natural gas), ammonia, and acids. In fact, hydrogen forms more compounds than any other element.
Molecular Hydrogen Properties
Hydrogen is a gas that is very light due to its low density and is used instead of helium to inflate weather balloons. It is colorless, odorless, and almost insoluble in water. Hydrogen forms diatomic molecules H2, where the two atoms are joined by a very strong covalent bond.
Under normal conditions, hydrogen is not very reactive because of the strength of the H-H bond. As a result, many reactions are slow, or require high temperatures or catalysts (often transition metals).
The hydrogen molecule is very stable and shows little tendency to dissociate at normal temperatures, since the dissociation reaction is highly endothermic. However, at high temperatures, in an electric arc or under ultraviolet light irradiation, H2 dissociates.
Atomic hydrogen is a strong reducing agent, and is commonly prepared in solution using a zinc-copper or mercury-aluminum pair.
Hydrogen reacts directly with most elements under appropriate conditions.
Hydrogen reacts with halogens.
Several metals react with H2, forming hydrides.
Large amounts of H2 are used in the industrial production of ammonia.
Hydrogen is also used to reduce nitrobenzene to aniline (in the dye industry), and in the catalytic reduction of benzene with CO to form methanol.
Hydrogen Isotopes
Isotopes are atoms of the same element that have different mass numbers. The difference in mass numbers arises from the different number of neutrons in the nucleus. Hydrogen found in nature consists of three isotopes: protium 11H or H, deuterium 21H or D, and tritium 31H or T. These isotopes contain in the nucleus 1 proton and zero, 1, or 2 neutrons, respectively. Protium is the most abundant.
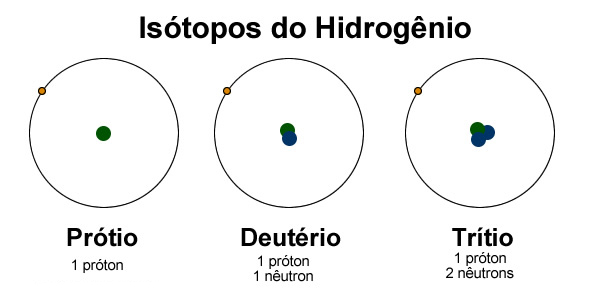
Hydrogen found in nature contains 99.986% of isotope 11H, 0.014% of isotope 21H, and 7 x10-16% of isotope 31H, so the properties of hydrogen are essentially due to the lightest isotope.
These isotopes have the same electronic configuration and essentially the same chemical properties. The only differences are found in reaction rates and equilibrium constants.
The Hydrogen Ion
The ionization energy is a very large amount of energy. Consequently, the bonds formed by hydrogen in the gas phase are generally covalent. Hydrogen fluoride is the compound most likely to contain an ionic hydrogen (H+), since it has the greatest difference in electronegativity. But even in this case, the bond has only 45% ionic character.