The Periodic Table currently adopted worldwide follows standards established by IUPAC (the International Union of Pure and Applied Chemistry), but its essential development involved the work of many people over many years. Although the Russian chemist Dmitri Mendeleev is often cited as the inventor of the Periodic Table, other scientists before him had already been trying to devise a classification system for the chemical elements.
Elements such as silver, gold, copper, and lead were known since ancient times, but the first scientific discovery of an element only occurred in 1669, when the alchemist Henning Brand discovered phosphorus. Over the following 200 years, dozens of other elements were found in nature. This created the need to organize them, and scientists began searching for properties that could serve as criteria for classification.
Dalton and atomic masses

The first attempt at organization was made in the early 19th century by the English chemist and physicist John Dalton. At that time, approximate values of the atomic masses of some elements had already been established.
Dalton listed the known elements in increasing order of atomic masses, describing the properties of each one and the compounds formed by them. However, this classification did not make sense, as it placed elements with very similar properties far apart from one another. The values of the atomic masses themselves were questionable, since Dalton calculated them based on imprecise data. The scientist took hydrogen, which had been experimentally verified as the lightest known element, and assigned it a “notional relative weight” of 1. The masses of the other elements were established relative to hydrogen, and this method resulted in many errors.
Döbereiner’s triads
In 1829, it was the German chemist Johann Wolfgang Döbereiner’s turn to contribute to science. Döbereiner analyzed the elements calcium, strontium, and barium and noticed that the mass of the strontium atom corresponded approximately to the average of the atomic masses of calcium and barium. He observed that this relationship also occurred in other triads, such as sulfur/selenium/tellurium and chlorine/bromine/iodine.
Döbereiner was the first scientist to relate the known chemical elements based on a given criterion; however, his observations were not considered relevant by the scientific community at the time. One flaw in his method was that many metals could not be grouped into triads.
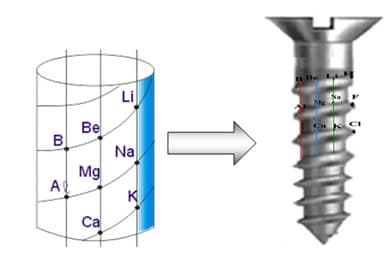
Chancourtois’s telluric screw
Later, in 1862, the French geologist Alexandre Chancourtois proposed the model known as the telluric screw. On a spiral drawn on the outer face of a cylinder, he arranged the chemical elements in increasing order of atomic masses. The cylinder was divided by vertical lines into 16 bands, so that elements with similar properties appeared one above the other within these bands. This model related the properties of the chemical elements to the positions they occupied in the sequence. The problem was that there were elements which, despite being in the correct position in the increasing order, displayed properties different from the other elements located in the same band, which invalidated the pattern. For this reason, the telluric screw attracted little interest.
Newlands’s Law of Octaves
Another idea came from the English chemist John Alexander Newlands, who took inspiration from music. It is known that in an ascending sequence of seven notes starting at C, the eighth note is again C, and the sequence then repeats. In 1864, Newlands devised a similar periodicity to be applied to the chemical elements. He lined up the elements known at the time in horizontal rows—seven in each row—in increasing order of atomic masses. The rows were positioned one above another. The first element of each row was the eighth relative to the previous row and had the same properties as the first element of that previous row. The same occurred with the second element, the third, and so on. In this classification, every eight elements the properties repeated; hence Newlands’s proposal became known as the Law of Octaves.
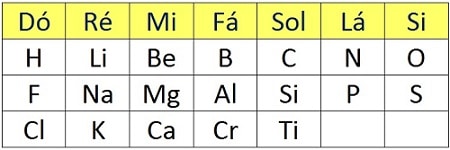
However, the model was coherent only up to calcium and did not hold for the elements that came after it in increasing order of atomic masses. The attempt to associate chemistry with music earned Newlands the scorn of the Chemical Society of London. Nevertheless, today he is recognized as the scientist who brought the notion of periodicity to chemistry, and his work is considered a precursor to Mendeleev’s.
Mendeleev’s Periodic Table of the Elements

Dmitri Ivanovich Mendeleev was a Russian chemist known as the father of the Periodic Table. His dedication to systematizing the chemical elements began in 1860, when he started grouping elements according to their common properties. By then, it was already known that elements had different atomic masses, and arranging them in increasing order of atomic masses was common. But Mendeleev believed that was not the whole story. At one point, after spending several days exhaustively reviewing all his chemical knowledge and the systematization attempts already made by other scientists, he began writing the elements while grouping them according to the chemical properties they exhibited. The problem was that this classification grouped elements whose atomic masses were very far apart.
Even as he felt discouraged knowing that the organizational pattern he observed in his attempt did not apply to all the elements known at the time, Mendeleev sensed that such a pattern did not emerge by chance. After all, Chancourtois (who proposed the “telluric screw”) had also perceived a pattern and had also run into the same problem: the fact that it did not apply to all elements. Mendeleev trusted Chancourtois’s chemical knowledge and did not believe he could be wrong; so he continued following the same clues, but without success.
Things began to become clearer for Mendeleev when he had the idea of associating the classification of elements with his favorite card game: solitaire. He took a set of paper cards and wrote on each one the name of an element, along with its atomic mass and chemical properties. Once the “deck” of chemical elements was ready, Mendeleev started arranging the cards as in solitaire: elements with similar chemical properties were like cards of the same suit, and within each “suit,” the increasing order of atomic masses was like the increasing numerical order of the cards. Ironically, he called this game “chemical patience,” not knowing he would indeed need a lot of patience in his journey. After arranging the cards, he realized his intuition was leading him in the right direction, but even so the “chemical patience” was imperfect. Then, exhausted, he fell asleep at his desk and had a dream. “I saw in a dream a table where all the elements fell into place as required. On awakening, I immediately wrote it down on a piece of paper,” Mendeleev later recounted.
The dream showed him how to fit together the knowledge he already had but could not consciously articulate. Upon waking and putting on paper what he had dreamed, Mendeleev perceived the logic behind the scheme: when the elements are listed in increasing order of atomic masses, the chemical properties they exhibit repeat periodically. For this reason, he called the model the Periodic Table of the Elements.
Two weeks after making the discovery, Mendeleev presented it to the scientific community by publishing an article entitled “A Suggested System of the Elements.” The year was 1869. At first, the Table listed the elements vertically in increasing order of atomic masses, and horizontally grouped them according to their chemical properties.

The Periodic Table, in a way, incorporated earlier models proposed by Döbereiner, Chancourtois, and Newlands, even if the validity of those patterns held only for certain parts of the Table. In addition, it accommodated all the elements known at the time. But Mendeleev still noticed inconsistencies in his model. One involved elements that, although in the same group as other elements with similar properties, had atomic masses that did not fit the increasing order. In such cases, Mendeleev challenged science by arguing that the problem did not lie in his classification system but in the calculation of the element’s atomic mass, which was wrong.
Another apparent flaw of the Periodic Table was the absence of elements with certain atomic mass values necessary for the continuity of the increasing sequence. For this problem, he adopted a simple but bold solution: he left gaps corresponding to them and continued the sequence with the known elements. He was certain that the elements corresponding to the gaps existed; they just had not been discovered yet. He was so confident that he even ventured to predict the properties of some of those unknown elements, basing his guesses on the atomic mass they should have and the position of the gap in the Table.
Meyer’s Periodic Table
Thanks to the speed with which he published his classification proposal, Mendeleev became known as the creator of the Periodic Table. But the truth is that a few years before him, another scientist had developed a very similar model. In 1864, the German chemist Julius Lothar Meyer studied the relationship between the elements’ masses and atomic volumes and constructed a graph based on these two quantities. From this study, Meyer devised a periodic classification of the elements, taking into account the properties they exhibited. His line of investigation was very close to Mendeleev’s, and the results obtained by the two were quite similar.
So why was Meyer’s work overshadowed by Mendeleev’s, who only reached consistent conclusions five years later? The answer is simple: because Meyer doubted his conclusions. The German chemist spent a long time reviewing his results and only published them about a year later. Moreover, after publication, Meyer hesitated in the face of questions from the scientific community, which cast doubt on the apparent disorder of the elements, the misplacement of some elements within their groups, and the lack of elements needed to make the scheme coherent. Mendeleev, on the other hand, was able to challenge established knowledge and defended his discovery with conviction.
Despite the firmness with which Mendeleev defended his Periodic Table, the scientific community was not convinced that the model was entirely correct, as there were obvious inconsistencies. One was the position of certain elements with very similar atomic mass values but very different properties. Problems like this led scientists to suspect that atomic mass might not be a suitable variable to serve as the organizing criterion for the elements.
Moseley and atomic numbers

In the early 20th century, around 1913, the English physicist Henry Gwyn-Jeffreys Moseley examined the characteristic X-ray spectra of about 40 elements. In this study, he discovered that all atoms of a given chemical element had identical nuclear charge, indicating that they possessed the same number of protons in their nuclei. The number of protons an element has in its nucleus corresponds to its atomic number. He observed that when the elements were placed in increasing order of atomic numbers, their properties repeated periodically.
It didn’t take long for Moseley to conclude that atomic number could be used as the criterion for organizing the chemical elements instead of atomic mass. Applying this standard corrected the flaws in Mendeleev’s and Meyer’s tables. The few gaps that remained in the Table were later filled by elements discovered or synthesized in the laboratory. Thus we arrived at a version of the Periodic Table very similar to the one we have today, composed of rows called periods (or levels) and columns called families (or groups).
Periods of the Periodic Table (or levels) – These are the horizontal rows. There are seven periods, numbered from top to bottom. The number of elements in each period varies widely: the first has only two elements, while the sixth has 32. That is because it counts the 14 elements of the lanthanide series, even though this series is shown below the main table as a separate block. The same happens with the seventh period, which has 32 elements, 14 of which are shown separately in the actinide series.
Families of the Periodic Table (or groups) – These are the vertical columns. They were once designated by Roman numerals and letters, but today IUPAC recommends numbering them simply from 1 to 18, from left to right.
Since Moseley’s contribution, atomic number has been consolidated as the basic criterion of the Periodic Table, valid to this day. The Table has been altered only by the addition of elements discovered or synthesized and by adjusting atomic mass values when more precise values are obtained.
Seaborg and the transuranium elements

The last major change applied to the Periodic Table resulted from the work of Glenn Theodore Seaborg. As part of the Manhattan Project, which worked to develop the atomic bomb, Seaborg headed the division dealing with transuranium elements (that is, elements with atomic numbers greater than 92, the atomic number of uranium). Together with E. M. McMillan, J. W. Kennedy, and A. C. Wahl, the American scientist discovered plutonium. He later discovered four other transuranium elements and took part in the discovery of five more.
In 1944, Seaborg hypothesized that elements with atomic numbers greater than that of actinium (which is given as 98) formed a series of elements similar to the lanthanide series. Based on this hypothesis, it was possible to explain the chemical properties of some elements already known and even those of others not yet identified. In 1945, he published a version of the Periodic Table that included the recently discovered transuranium elements. The configuration of this Table differed from the previous one by placing the actinide series below the lanthanide series. In 1951, Seaborg received the Nobel Prize in Chemistry. Element 106 in the Periodic Table is called seaborgium in his honor.